Schoffelen & Plasqui explained the methods for indirect calorimetry system validation in their 2017 paper: “Classical experiments in whole-body metabolism: open-circuit respirometry—diluted flow chamber, hood, or facemask systems”.
Validation differs from calibration
It is crucial to do routine validity testing when utilizing a calorimetry system. Calibration should not be confused with validity testing. First, even while the calorimeter’s various parts, like the flowmeter, pressure sensor, and analyzer, may have been calibrated and proven adequate for their intended application, this does not assess how well the system as a whole works. Before measuring a subject, the analysers should, secondly, be calibrated daily or even more frequently. This can be accomplished by sequentially passing two gases through the analysers that have known amounts of O2 and CO2 in them (dual span), one of which may be 100% N2 gas (zero for O2 and CO2), or by using a single gas and room air. The latter makes the assumption that the ambient air contains an usual 20.9% oxygen content and typically filters out the 0.04% CO2 to produce a zero gas for CO2. In addition to this analyzer calibration, the following choices are available and necessary to evaluate the system’s validity.
Alcohol combustion
The combustion of a measured amount of a high-purity fuel, like methanol or ethanol, is a classic indirect calorimeter validation technique. Such a fuel will burn in one gram with a given amount of oxygen required and a known amount of carbon dioxide produced. The amount of fuel burned can then be determined by comparing measured gas-exchange values from the calorimeter to known values based on the chemical process. The most popular technique involves burning methanol or high-purity ethanol within the calorimeter using an alcohol burner (Fig. 7). To accommodate the blazing flame and its heat, a different, more heat-resistant hood is used in hood systems in place of the normal one. This method puts the calorimeter’s capacity to handle both produced heat and H2O to the test.
Fig. 7
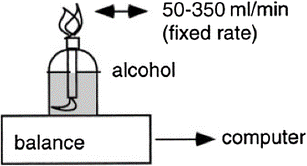 Alcohol combustion may be used to validate indirect calorimeters. The amount of alcohol burned is determined by the burner’s weight change. The rate of combustion can be continuously measured by connecting the balance to a computer.
Alcohol combustion may be used to validate indirect calorimeters. The amount of alcohol burned is determined by the burner’s weight change. The rate of combustion can be continuously measured by connecting the balance to a computer.
Gas infusion
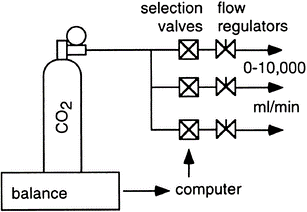
A measured amount of a high-purity gas is infused as part of a second method, and the apparatus’ gas-exchange results must match the amount infused. Despite the possibility of cooling due to adiabatic expansion, this process.
Fig. 8
Gas infusion can be used to validate indirect calorimeters. The amount of gas injected is determined by volumetric flow or weight change. A computer might allow for ongoing registration and adjustments to the infusion rate.
It is easy to understand how injected gas and gas-exchange measurements match up for CO2. While a quantity of CO2 is delivered into the calorimeter that must precisely match that produced by a subject register and is accounted for, the O2 intake must stay zero.
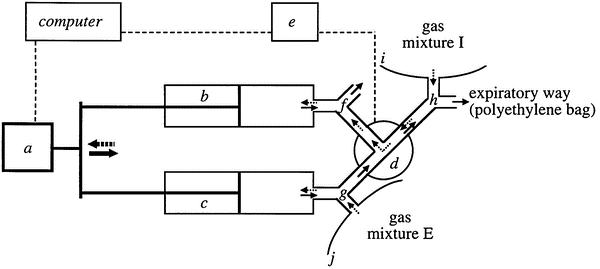
Simulating oxygen consumption is more challenging since there cannot be an infusion of “negative” oxygen to simulate oxygen intake. Instead, the amount of oxygen in the exhaust airstream is reduced by the introduction of an inert gas, often N2. Gas infusion is the recommended method for calorimeters that use a breath-by-breath analysis, measuring inhalation and exhalation separately. In that case, the calorimeter may not even function in this mode without the infusion and a correct lung simulation for breathing (Fig. 9) (Prieur et al. 1998).
Fig. 9
Gas-exchange simulation system diagram. Gas flow during inspiration is shown by dotted arrows, while gas flow during expiration is shown by solid arrows. Motion control, 31 syringes, pneumatic directional control valve, automated controller for directional valve, two-way non-rebreathing valves, and I and J polyethylene bags are all included in the motion control chain. Adopted from: a method for simulating human gas exchange to regulate the accuracy of metabolic measurements (Prieur et al. 1998). Unfortunately, it is difficult to find a combined gas infusion and accurate lung simulator, which largely prevents routine on-site validation of breath-by-breath calorimetry. Instead, only a portion of such calorimeters can be tested in their hood mode. The ability to achieve the widest range of gas concentrations and quick modulation of infused gas quantities are two benefits of gas infusion in general. As a result, it is the preferred technique for imitating exercise.
Parallel validation
By merging two methods—one previously verified system and the system under test—a third validation technique is created. The two systems are linked in order to measure identical samples, and the results are contrasted. It is imperative to use extreme caution when implementing this parallel validation to avoid system interference. This needs to be demonstrated, together with the continued validity of the earlier certified system. It is insufficient to just link two devices, one of which has previously been validated in the literature. A calorimeter used as a reference in this test needs to be validated locally because it cannot be assumed to function as a “gold standard” device without first evaluating each component’s performance individually and taking into account potential interactions.
A participant in the parallel measurement will often be used in parallel validation to introduce the idea of biological repeatability.
Validation through biological reproducibility
Consider a technically sound calorimeter that fails to demonstrate consistency for healthy, normal participants within predetermined biological variability limitations. In that situation, the calorimeter is inappropriate since the technological validation approach may not have adequately simulated biology. It cannot be regarded as valid. Consider a calorimeter that is reproducible for healthy individuals in the typical range, but whose technical validity or “level” of measured value is unknown. The calorimeter may or may not be accurate in this situation. It is also ineligible for validation. A calorimeter can only be regarded technically validated if it also demonstrates consistency for normal healthy persons within established limits of biological variability. It must have demonstrated its capacity to replicate measurements with participants and its ability to quantify EE at precise levels.
From the lowest level of EE, i.e., SMR (Schoffelen and Westerterp 2008), BMR (Adriaens et al. 2003), until 24-h EE (Schoffelen et al. 1997), to the highest level possible, i.e., VO2max (Schoffelen et al. 2017), repeated measurements with subjects were carried out to test reproducibility (including both technical and biological variation) (Table 2). The CV% for high-intensity exercise was the least and the biggest for the categories of sleep, OMR, BMR, 24-h EE, and exercise (Table 2). Exercise has the highest standard deviation, measured in kJ min1, although having the lowest CV% (1.2%). In contrast, SMR had the highest absolute repeatability with a median CV% of 2.4%, closely followed by OMR, 24-h EE, and even BMR using an outpatient methodology (Table 2).
Table 2 Reproducibility of EE measures using repeated subject testing
| Category | EE (kJ min−1) | SD (kJ min−1) | CV (%) |
| SMR (room) | 4.6 | 0.11 | 2.4 |
| OMR (room) | 4.8 | 0.13 | 2.8 |
| BMR (hood) | 4.7 | 0.15 | 3.3 |
| 24-h EE (room) | 6.8 | 0.13 | 1.9 |
| maximal exercise, VO2max | ~ 90 | ~ 1 | 1.2 |
Related products
Whole body room calorimeters
The Room Calorimeter offers the highest validated accuracy and reproducibility in the market. Designed on a system level out of the highest quality components, this is the gold standard for energy expenditure studies of any kind; 24-hr energy expenditure, high intensity exercise testing and many more. Validated and applied in 100’s of research studies.
Omnical
The Omnical is the market’s most versatile and accurate indirect calorimeter for research. It allows users to conduct investigations in a variety of study sectors using cutting-edge technology and top-of-the-line precision measurement devices. The system is designed to monitor energy metabolism with great precision, ranging from resting metabolic rate (RMR) through sports performance testing (e.g. VO2max tests).
How can we help you with your research?
Maastricht Instruments creates equipment in the field for indirect calorimetry measurements. We provide support for studies, research and measurements alongside our indirect calorimetry products.
Consult us about our indirect calorimetry metabolic cart, whole room calorimeter systems or accelerometry add-ons. Please contact us or find more information on our information pages.
Reference
Schoffelen, P.F.M., Plasqui, G. Classical experiments in whole-body metabolism: open-circuit respirometry—diluted flow chamber, hood, or facemask systems. Eur J Appl Physiol 118, 33–49 (2018). https://doi.org/10.1007/s00421-017-3735-5