Introduction
Metabolic health is intricately linked to the dynamic interplay between carbohydrate and fat oxidation rates. During periods of carbohydrate intake, the release of insulin by the pancreatic β-cells orchestrates the uptake of carbohydrates by various organs, including skeletal muscle, liver, and adipose tissue, leading to augmented whole-body carbohydrate oxidation and suppressed fat oxidation. Conversely, in the absence of carbohydrate intake during the nocturnal period, glucagon levels rise, triggering adipose tissue lipolysis and promoting heightened fat oxidation.
Notably, recent investigations by the researchers have revealed notable discrepancies in nocturnal substrate oxidation patterns between older individuals with metabolic compromise and their young, lean counterparts. Surprisingly, the older group exhibited reduced rates of nocturnal fat oxidation and elevated rates of nocturnal carbohydrate oxidation, indicating an inability to achieve a genuine nocturnal fasting state despite comparable preceding daytime activities and controlled food intake. Notwithstanding, disentangling the individual contributions of age, overweight/obesity, and slightly elevated glucose levels in the older group has proven challenging.
While impaired metabolic inflexibility is well-documented in individuals with overweight, obesity, insulin resistance, and non-alcoholic fatty liver disease (NAFLD), elucidating their distinct influences on nocturnal substrate oxidation remains largely unexplored. Additionally, the complex interrelationships among these factors further complicate our understanding.
To address these knowledge gaps, the researchers conducted a rigorous analysis using baseline data from 10 human clinical trials with homogeneous study designs. The overarching aim is to discern the primary participant characteristics that exert the greatest influence on the variations observed in nocturnal substrate oxidation. By unraveling the underlying factors governing metabolic inflexibility, this study seeks to advance the comprehension of metabolic health in older individuals.
Goal of this study
The goal of this study conducted by the researchers is to unravel the underlying factors that contribute to the observed differences in nocturnal substrate oxidation between populations. Specifically, the study aims to investigate whether baseline participant characteristics can explain the variations in nocturnal substrate oxidation. To achieve this, the researchers analyze baseline data from multiple human clinical trials with similar study designs, focusing on the placebo or control arms. By comprehensively examining participant characteristics and their associations with nocturnal substrate oxidation, the study seeks to shed light on the complex interplay between age, overweight/obesity, and glucose levels in metabolic compromise. Ultimately, the findings of this research endeavor have the potential to enhance our understanding of metabolic health and guide the development of targeted interventions tailored to individuals’ specific characteristics, paving the way for personalized approaches to optimize metabolic well-being.
Methods
Participants and Study Design: The researchers combined data from 10 human clinical trials that were approved by the Ethics Committee of Maastricht University Medical Center and registered at clinicaltrials.gov and trialregister.nl. The total sample consisted of 124 participants, categorized as follows: 23 young lean individuals (aged 18-30 years, BMI < 25), 10 older lean individuals (aged 40-75 years, BMI < 25), 44 older individuals with overweight or obesity but without type 2 diabetes (aged 40-75 years, BMI ≥ 25), and 47 older individuals with overweight or obesity and type 2 diabetes (aged 40-75 years, BMI ≥ 25 and diagnosed with type 2 diabetes).
Indirect Calorimetry: Oxygen consumption and carbon dioxide production were continuously measured during the night using whole-chamber indirect calorimetry. Energy expenditure and substrate oxidation were calculated using the Brouwer equation, while protein oxidation was estimated using the Weir equation. The respiratory exchange ratio (RER) was determined by dividing carbon dioxide production by oxygen consumption. Energy expenditure, RER, and substrate oxidation were calculated for the entire night and specific time intervals to examine the temporal pattern of substrate oxidation.
Hepatic Lipid Content: Hepatic lipid content was quantified using proton magnetic resonance spectroscopy (1H-MRS) on a 3T MRI scanner. Liver fat spectra were acquired using different protocols, and the lipid content values were reported as a T2 corrected ratio of the CH2 peak relative to the sum of the unsuppressed water peak and the CH2 peak, converted to weight/weight percentage.
Results
Baseline Characteristics among Different Populations: The study comprised 124 participants distributed across four groups: young lean (n=23), old lean (n=10), overweight (n=44), and type 2 diabetes (n=47). Significant differences were observed in age and BMI, with the young lean population being the youngest and leanest. The overweight population had a higher proportion of females. Fasting plasma glucose levels were highest in the type 2 diabetes group, along with a greater liver fat percentage. Dinner time was significantly earlier in the old lean population.
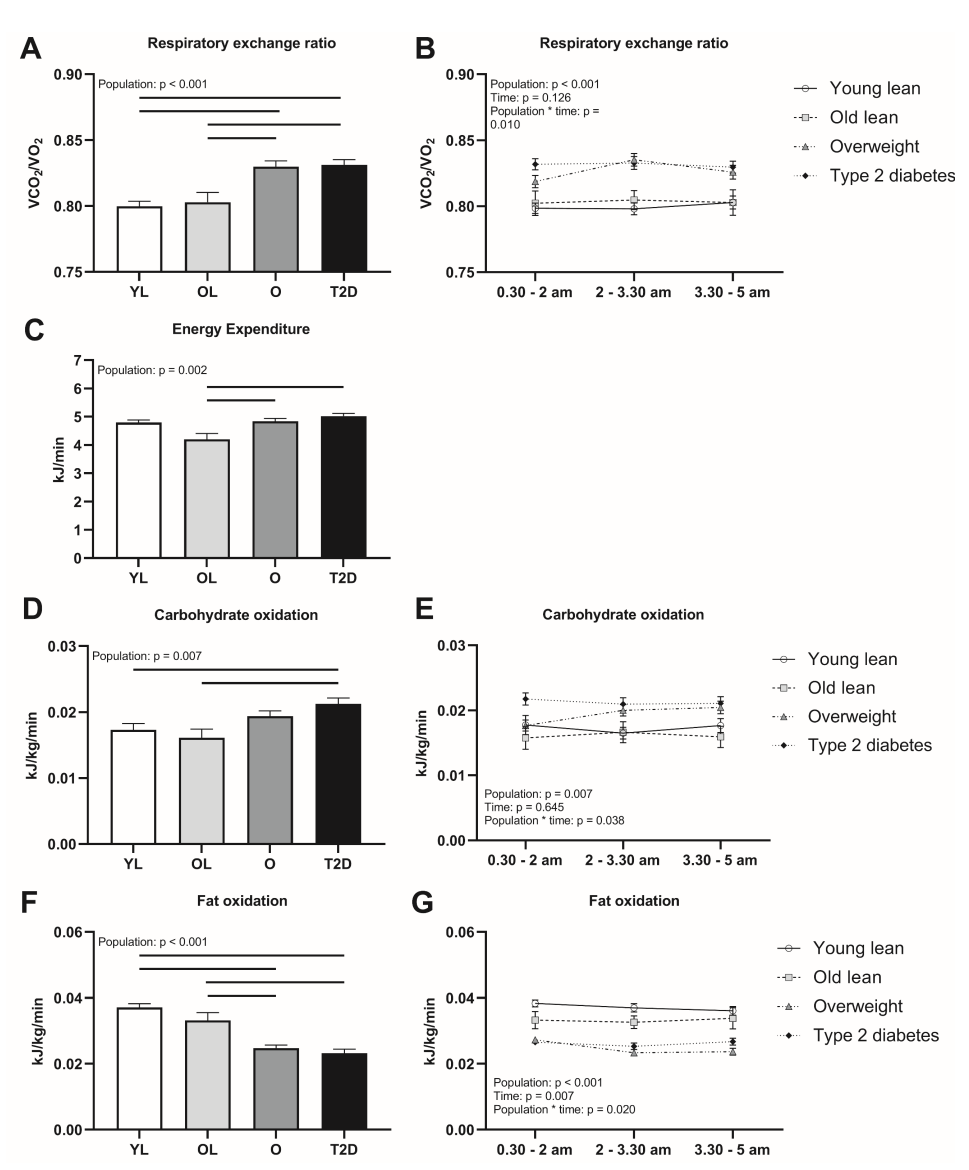
Nocturnal Energy Expenditure and Substrate Oxidation among Different Populations: Analysis revealed distinct variations in nocturnal respiratory exchange ratio (RER) among the populations. The young lean and old lean populations exhibited lower RER compared to the overweight and type 2 diabetes groups. Energy expenditure was also significantly different, with the old lean population demonstrating lower levels. Carbohydrate oxidation was higher in the type 2 diabetes group compared to the lean populations, while no statistical difference was observed in the overweight population. Fat oxidation was lower in the overweight and type 2 diabetes groups compared to the lean populations.
Temporal Patterns of Substrate Oxidation: Temporal analysis showed that the overweight population had higher carbohydrate oxidation and lower fat oxidation in the initial part of the night. No significant differences were found between the lean populations or between the type 2 diabetes and overweight groups.
Baseline Characteristics and Nocturnal Substrate Metabolism: Correlations indicated that age and BMI were positively associated with nocturnal RER and carbohydrate oxidation. Fasting glucose showed a positive trend with RER but did not reach statistical significance. No significant correlations were observed between RER or substrate oxidation and liver fat percentage. The removal of young individuals eliminated the correlation between age and nocturnal RER. Fat oxidation displayed a negative correlation with age and a positive correlation with BMI. No significant associations were found between fat oxidation and fasting glucose or liver fat percentage.
Sex Differences in Nocturnal Substrate Metabolism: There were tendencies for differences in RER between males and females, with higher RER in overweight males compared to females. No significant variations were observed in carbohydrate oxidation or fat oxidation between males and females in any population, although there was a trend toward higher fat oxidation in males within the old lean group.
Figure Explanation:
Figure 1 depicts the nocturnal respiratory exchange ratio (RER), energy expenditure, and substrate oxidation in different populations. Panel A shows the overnight RER in each population, indicating the relative contribution of carbohydrate and fat oxidation. Panel B illustrates the RER over the course of the night for each population, revealing any changes in substrate utilization patterns. Panel C displays the overnight energy expenditure in the different populations. Panels D and E represent overnight carbohydrate oxidation, with panel D showing the overall levels and panel E presenting the pattern over the night. Similarly, Panels F and G represent overnight fat oxidation, with Panel F displaying the overall levels and Panel G showcasing the pattern throughout the night. The sample sizes (n) for each population are specified. Mean ± SEM is used to present the data. The lines between populations indicate significant differences. The abbreviations used are: O (overweight), OL (old lean), T2D (type 2 diabetes), and YL (young lean).
Conclusion
Switching from high carbohydrate oxidation rates during the day to higher fat oxidation rates during the night is indicative of good metabolic health (1). In our recent study, we found that metabolically compromised individuals, including those who are older, overweight or obese, and have higher fasting plasma glucose levels, exhibited higher rates of nocturnal carbohydrate oxidation and lower rates of fat oxidation compared to young healthy lean individuals. To identify the factors contributing to this lower nocturnal fat oxidation, we combined data from 10 human clinical trials. Our analysis revealed distinct differences in nocturnal substrate oxidation among the populations studied. The overweight and type 2 diabetes population exhibited higher carbohydrate oxidation and lower fat oxidation rates compared to the young and old lean populations. Interestingly, the old lean population demonstrated a nocturnal respiratory exchange ratio (RER) similar to the young lean population, suggesting that age is not the primary driver of higher nocturnal RER observed in older overweight and/or type 2 diabetes populations. Notably, higher body mass index (BMI) was strongly associated with lower nocturnal fat oxidation. The underlying mechanisms linking BMI to altered substrate oxidation in overweight and obese individuals, with or without type 2 diabetes, warrant further investigation. While our study provides initial insights into the predictors of nocturnal substrate oxidation, future research with larger and more extensively phenotyped study populations is needed to validate and expand upon these findings. In conclusion, overweight individuals and patients with type 2 diabetes exhibit lower nocturnal fat oxidation, and in the case of type 2 diabetes patients, increased nocturnal carbohydrate oxidation. Age is not the primary determinant of the higher respiratory exchange ratio observed in older overweight populations. BMI emerges as a significant factor associated with altered substrate oxidation during the night. Further investigations are required to ascertain the role of BMI-associated metabolic alterations in influencing substrate oxidation patterns in overweight and obese individuals with and without type 2 diabetes.
Related products
Whole body room calorimeters
The Room Calorimeter, used in numerous research studies, is spotlighted for its unparalleled accuracy and reproducibility in measuring energy expenditure in various contexts. This tool is vital for generating reliable data in studies exploring energy expenditure during various activities, from 24-hour energy expenditure evaluations to high-intensity exercise testing.
How can we help you with your research?
Maastricht Instruments creates equipment in the field for indirect calorimetry measurements. We provide support for studies, research and measurements alongside our indirect calorimetry products. Consult us about our indirect calorimetry metabolic cart, whole room calorimeter systems or accelerometry add-ons. Please contact us or find more information on our information pages.
Reference
Veelen, A. H. (2023). Improving flexibility in substrate metabolism: a pharmacological and lifestyle approach. [Doctoral Thesis, Maastricht University]. Maastricht University. https://doi.org/10.26481/dis.20230125av