Introduction
Light is the primary zeitgeber, or time cue, for synchronizing the internal biological clock of mammals with the 24-hour light-dark cycle. The suprachiasmatic nucleus in the hypothalamus acts as a master pacemaker, integrating light-dark information with endogenously generated rhythms to synchronize peripheral clocks in organs like the liver, skeletal muscle, and pancreas. This ensures anticipation of metabolic demands associated with specific time slots within the light-dark cycle, such as feeding, fasting, and physical activity. Optimizing indoor lighting to mimic natural light conditions promotes regulation of behavioral rhythms, sleep/wake cycles, and metabolic health.
However, modern society’s pervasive artificial light disrupts this synchronization. People expose themselves to electrical light and screens during the dark period, leading to detrimental effects known as light at night (LAN). LAN exposure increases postprandial glucose and insulin levels, and is linked to higher incidence of type 2 diabetes. Additionally, inadequate daytime bright light exposure is prevalent, with most time spent indoors under lower light levels compared to natural daylight. Bright light exposure before and during breakfast affects glucose and lipid metabolism.
Goal of this study
In this study, researchers investigated whether optimizing artificial light exposure over 24 hours improves whole-body energy and substrate metabolism, and glucose homeostasis in individuals with insulin resistance. Specifically, they hypothesized that combining bright light during the daytime and dim light in the evening would yield more favorable metabolic effects compared to the reverse combination. Through a randomized crossover design, 24-hour metabolic phenotyping using indirect calorimetry and frequent blood sampling for triglycerides, glucose, insulin, and melatonin concentrations was performed. This study’s findings will provide insights into the potential benefits of optimized artificial light exposure on metabolic health in individuals with insulin resistance.
Methods used
The study employed a randomized crossover design with two arms and received approval from the Medical Research Ethics Committee of Maastricht University Medical Center. Written informed consent was obtained from all participants, and the study adhered to the Declaration of Helsinki. The research took place between July 2018 and November 2019, with registration in ClinicalTrials.gov under the number NCT03829982.
Male and female overweight volunteers aged 40-75 years with insulin resistance were recruited. Insulin resistance was defined based on impaired fasting glucose, impaired glucose tolerance, HbA1c levels, or low insulin sensitivity. Participants were non-smokers, generally healthy, and maintained a consistent bedtime of 23:00 h ± 2 h with 7-9 hours of sleep per day. Exclusion criteria included shift work, recent long-distance travel, (history of) cardiovascular diseases, and regular medication that could affect study outcomes. Participants with extreme early or late chronotypes were also excluded using the Morningness-Eveningness Questionnaire Self-Assessment Version 1.3.
The study comprised two 40-hour sessions, during which participants stayed overnight in a respiration chamber. In the Bright day-Dim evening session, participants experienced bright light (1250 lx) during the daytime (08:00-18:00 h) and dim light (5 lx) during the evening (18:00-23:00 h). In the Dim day-Bright evening session, participants were exposed to dim light (10 lx) during the daytime and bright light (1250 lx) during the evening. Actigraphy using an Actiwatch Spectrum monitored participants’ adherence to sleep times and compared activity patterns and sleep characteristics between home and the respiration chamber.
Energy metabolism and substrate oxidation were assessed through indirect calorimetry. Measurements of oxygen consumption and carbon dioxide production were collected using the Omnical system from Maastricht Instruments, allowing the calculation of daily energy expenditure, sleeping metabolic rate (SMR), and substrate oxidation. SMR, defined as the lowest 2-hour mean energy expenditure, was multiplied by an activity factor of 1.5 to determine energy requirements. Macronutrient composition of the provided meals consisted of approximately 55% carbohydrates, 30% fat (with 9% saturated fat), and 15% protein. Participants consumed breakfast, lunch, and dinner at specific times, and each meal had a standardized caloric and macronutrient content. Meals were completed within 20 minutes while seated at a table, with no additional snacks or drinks apart from water. The ambient temperature in the respiration chamber was maintained at 21°C, and light exposure was strictly controlled according to the assigned protocol.
Skin and core body temperatures were measured using wireless temperature sensors and a telemetric pill, respectively, to monitor thermoregulatory responses. Body composition, including body mass and volume, was determined using air-displacement plethysmography (BodPod).
Frequent blood sampling was conducted to analyze various metabolic parameters. Fasted blood samples were collected on days 2 and 3 at specific time points, and postprandial blood samples were obtained every 30 minutes for 4 hours after breakfasts and the second dinner. Triglyceride levels were measured in serum using a colorimetric assay, while plasma glucose levels were determined using a glucose analyzer. Insulin levels were measured via an enzyme-linked immunosorbent assay (ELISA) kit. Melatonin concentration was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Statistical analysis involved general linear mixed models and paired t-tests to evaluate differences between conditions. The significance level was set at α < 0.05 for all analyses. Mean ± SEM were used to present the data unless otherwise specified. GraphPad Prism 8 software was utilized for statistical analysis.
Results
participant compliance with instructions before and during the intervention was high. Actigraphy data showed no significant differences in bed and wakeup times or physical activity between the pre-period at home and the intervention period in the respiration chambers.
Distal skin temperature (mean of foot and hand temperatures) showed differences between the two lighting conditions. In the Bright day-Dim evening condition, distal skin temperature was lower at 18:00 h compared to the Dim day-Bright evening condition. After switching the light conditions at 18:00 h, distal skin temperature increased and was higher at 23:00 h in the Bright day-Dim evening condition. In contrast, in the Dim day-Bright evening condition, distal skin temperature decreased over the evening. The distal-proximal skin temperature gradient (DPG), an indicator of vasodilation, followed the same pattern as distal skin temperature.
Plasma melatonin levels were significantly suppressed in the Dim day-Bright evening condition compared to the Bright day-Dim evening condition, indicating the strong impact of evening light exposure on melatonin secretion.
Regarding postprandial glucose and triglyceride levels, the Bright day-Dim evening condition showed a trend towards increased plasma glucose after the first breakfast compared to the Dim day-Bright evening condition. Plasma triglyceride levels after the first breakfast were significantly higher in the Bright day-Dim evening condition. Additionally, plasma glucose levels before dinner were lower in the Bright day-Dim evening condition, and glucose levels after dinner increased more compared to the Dim day-Bright evening condition. Plasma insulin and triglyceride levels after dinner did not differ between conditions.
No significant differences were observed in plasma metabolites before and after breakfast on the last day of the intervention under standardized dim light conditions.
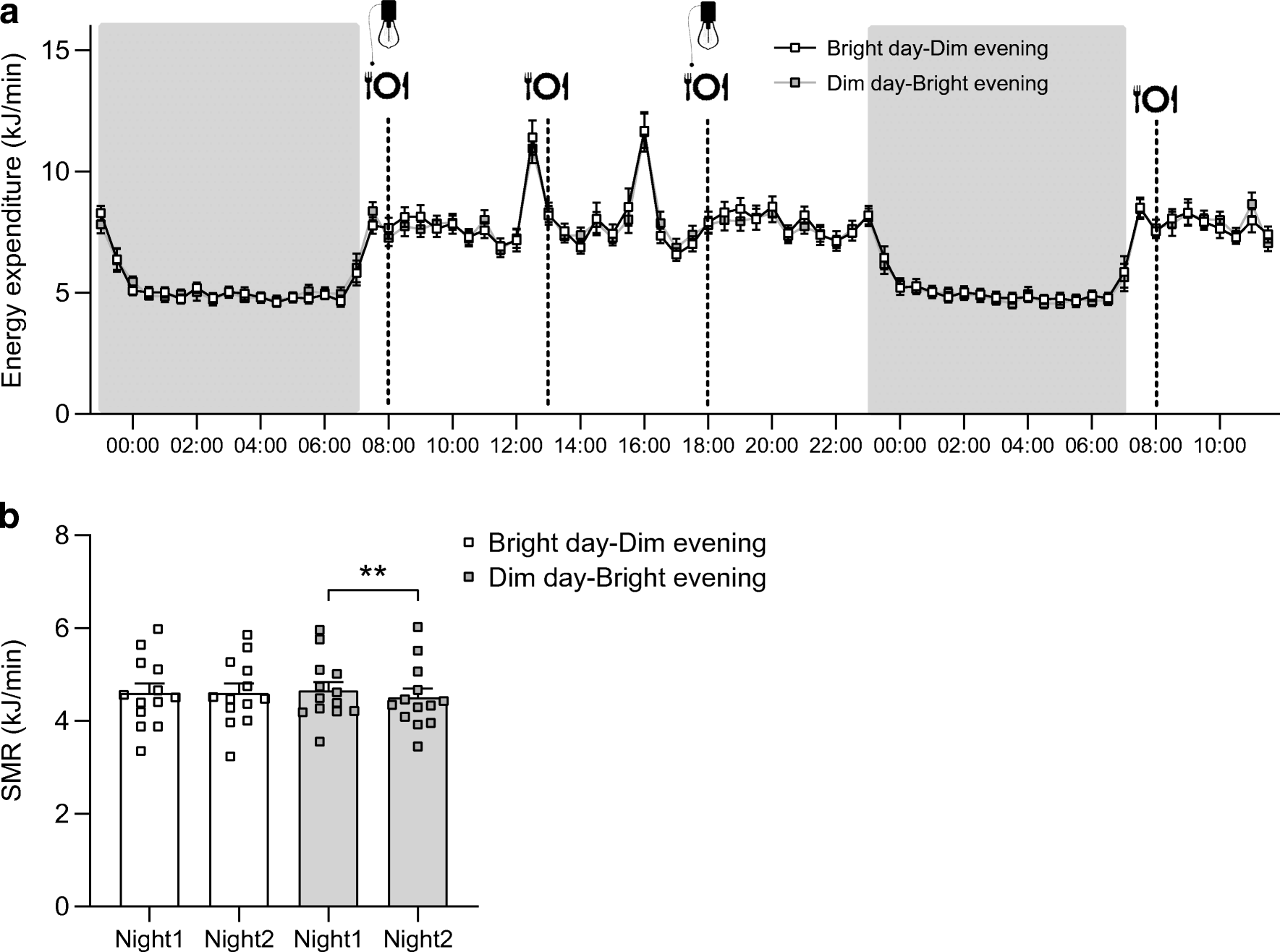
Energy expenditure and sleeping metabolic rate (SMR) were not significantly different between light conditions during the 24-hour period. However, after dinner, energy expenditure was higher in the Bright day-Dim evening condition. SMR was reduced in the night after the light intervention in the Dim day-Bright evening condition, while the SMR of the night after the light intervention was higher in the Bright day-Dim evening condition.
Figure Explanation: Energy expenditure over the entire time spent in the respiration chamber (a) and SMR of both nights per condition (b); p-values based on paired t-tests; **p<0.01. Energy expenditure did not significantly differ between conditions, indicating that light interventions had no substantial effect. Similarly, there were no significant differences in the sleeping metabolic rate (SMR) during both nights, suggesting that the metabolic rate during sleep was not influenced by the light conditions. These findings indicate that overall energy expenditure and SMR remained relatively consistent regardless of the specific light interventions.
Respiratory exchange ratio, a measure of substrate utilization, did not differ between the two lighting conditions.
Conclusion
In this study, the researchers investigated the effects of different lighting conditions on metabolic variables in insulin-resistant individuals. The findings suggest that optimizing indoor light conditions to mimic the natural light/dark cycle can have favorable effects on whole-body energy and glucose metabolism.
The researchers observed that spending the day in bright light and the evening in dim light resulted in lower plasma glucose levels before the last meal of the day, while plasma glucose levels after that meal were higher when consumed under dim light conditions. The Bright day-Dim evening condition also increased energy expenditure during dinner and maintained the sleeping metabolic rate compared to the Dim day-Bright evening condition. The suppression of melatonin secretion in the Dim day-Bright evening condition further highlights the influence of light exposure on metabolic processes.
Moreover, the researchers found that the light conditions influenced distal skin temperature and the distal-proximal skin temperature gradient, indicating changes in vasodilation and thermoregulation. The Bright day-Dim evening condition exhibited a diurnal pattern in distal skin temperature, which is associated with a healthy blood pressure variation.
Overall, this study provides valuable insights into the impact of light exposure on metabolic variables in insulin-resistant individuals. The findings underscore the potential of optimizing indoor lighting conditions to promote metabolic health and prevent the development of chronic diseases. Further research is warranted to explore the implementation of different light regimens in various settings to improve metabolic outcomes and enhance overall well-being.
Related products
Omnical
The Omnical is the most versatile and accurate indirect calorimeter for research purposes on the market. Comprised of state-of-the-art technology using the highest-class precision measurement instruments, it enables customers to perform studies in various research fields. The system is designed to measure energy metabolism ranging from resting metabolism rate (RMR) to sports performance testing (e.g. VO2max tests) with high accuracy.
How can we help you with your research?
Maastricht Instruments creates equipment in the field for indirect calorimetry measurements. We provide support for studies, research and measurements alongside our indirect calorimetry products. Consult us about our indirect calorimetry metabolic cart, whole room calorimeter systems or accelerometry add-ons. Please contact us or find more information on our information pages.
Reference
Harmsen, JF., Wefers, J., Doligkeit, D. et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day. Diabetologia 65, 721–732 (2022). https://doi.org/10.1007/s00125-021-05643-9